Analytical instrumentation
Thermometric Titration of Sodium, Aluminum and Fluorine
Jul 24 2008
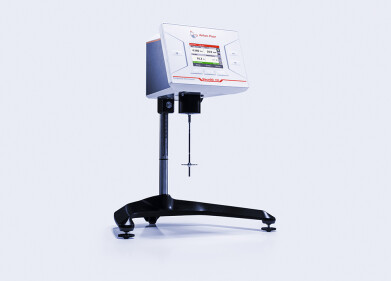
A typical example of a thermometric titration is the less well-known exothermic reaction in which the mineral elpasolite (NaK2AlF6) is formed from sodium, potassium, aluminum and fluoride ions. The industrial determinations of sodium, aluminum and fluoride are based on this reaction; these are all known as versions of the «elpasolite method».
With the aid of thermometric titration, the elpasolite method offers a quick, reliable and extremely accurate method for determining sodium, aluminum and fluoride ions in numerous different sample matrices. The 859 Titrotherm from Metrohm is the suitable favourably-priced instrument for these determinations.
Digital Edition
PIN 25.6 Buyers' Guide
January 2025
Buyers' Guide Directory - Product Listings by Category - Suppliers Listings (A-Z) Articles Analytical Instrumentation - ASTM D7042: The Quantum Leap in Viscosity Testing Technology -...
View all digital editions
Events
Jan 20 2025 San Diego, CA, USA
Jan 22 2025 Tokyo, Japan
Jan 25 2025 San Diego, CA, USA
SPE Hydraulic Fracturing Technology Conference and Exhibition
Feb 04 2025 The Woodlands, TX, USA
Feb 05 2025 Guangzhou, China